

Efficient QMS according to EN ISO 13485: Innovation despite MDR and shortage of skilled workers
MDR and a shortage of skilled workers are challenging companies - but an efficient quality management system (QMS) creates opportunities for ...

Automation and robotics for ATMPs
Robotics and automation offer groundbreaking manufacturing methods for ATMPs. ATMPs are achieving groundbreaking success in the treatment of ...

Do your methods comply with the new ICH requirements?
ICH Q2(R2) and ICH Q14 describe mandatory requirements for the development, life cycle and validation of analytical methods.

The first CRISPR/Cas9-based therapy has reached the market
The admission of Casgevy (Exagamglogene autotemcel) opens a new chapter in CRISPR/Cas9-based gene therapy and ATMP manufacture
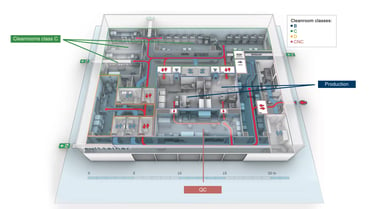
Transferring novel therapies successfully to patients and market
"ATMPs" – a long way from development to clinical trial – here's how to get there safely! ATMPs (advanced therapy medicinal products) are considered ...

Prohibition of PFAS – Evaluate, assess risks, time to act
Valicare experts present solutions to you Per- und Polyfluoroalkyl substances (PFAS) are widely used in the pharmaceutical and biotech industry as ...

Revision of Annex 1 - New approaches are required soon!
Get an overview about the most important upcoming changes The revision of Annex 1 of the EU GMP Guideline will be effective this year. It faces ...

SMEPAC Test to Measure the Tightness of Containment Systems
Working with highly active and hazardous drugs and ingredients requires evidence that the used containment systems are leak proof!

Computerized System Validation (CSV) – GAMP 5 Second Edition
2nd Edition of the GAMP 5 Guideline will address required changes in computerized system validation (CSV). Have you adopted the changes yet?

Celebrating 20 years of Valicare
From drug development to marketing authorization, Valicare supports chemical, pharmaceuticals and biotechnology companies around the globe.

New ICH Q14 Guideline: Analytical procedure development
Analytical procedure development: harmonization, development process as well as improvement of change management, approval and communication with ...

Revision of ICH Q9 (R1) Guideline on Quality Risk Management
Innovations in the implementation of risk management in the pharmaceutical industry: product availability, hazard identification, and attention to ...