

Automation and robotics for ATMPs
Robotics and automation offer groundbreaking manufacturing methods for ATMPs. ATMPs are achieving groundbreaking success in the treatment of ...

The first CRISPR/Cas9-based therapy has reached the market
The admission of Casgevy (Exagamglogene autotemcel) opens a new chapter in CRISPR/Cas9-based gene therapy and ATMP manufacture
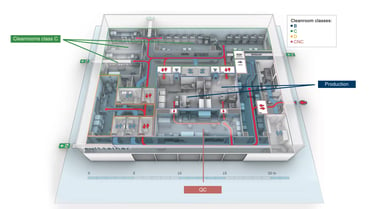
Transferring novel therapies successfully to patients and market
"ATMPs" – a long way from development to clinical trial – here's how to get there safely! ATMPs (advanced therapy medicinal products) are considered ...

Novelties by EMA on medicinal products with genetically modified cells
Updated EMA guideline for medicinal products containing genetically modified cells provides innovations, recommendations and improvements to be ...

FDA approves Breyanzi®, a new CAR-T cell therapy drug
Fourth approved CAR-T cell therapy soon to be available in Europe?
Vaccinations with mRNA-based medicinal products
How do mRNA-based medicinal products work and how safe are they?

MSC & Covid-19
In order to identify approaches to manage the current Covid-19 pandemic, the therapeutic use of mesenchymal stromal cells (MSCs) is increasingly ...

FDA opens up approval process for RYONCYL™
Mesoblast Ltd, headquartered in Australia with subsidiaries in the USA and Singapore, develops and distributes innovative allogenic cell therapeutics ...

FDA approves CAR T-cell therapy Tecartus™
On July 24, 2020, the US health authority FDA approved Tecartus™ (formerly KTE-X19) a cell-based gene therapy based on the chimeric antigen receptor ...

BioNTech SE acquires Neon Therapeutics
In mid-January the biotech company BioNTech SE based in Mainz announced the acquisition of the US-American T-cell therapy specialist Neon ...