GMP cleanroom modules for ATMP production
Valicare offers you a unique opportunity: In cooperation with the engineering and project management department of our parent company Syntegon, a globally active manufacturer of processing and packaging equipment, Valicare designs flexible cleanroom modules for the GMP-compliant development and production of starting materials through to investigational clinical trial ATMP products (iATMPs). These turnkey GMP-compliant cleanroom modules are specifically tailored to your individual requirements.
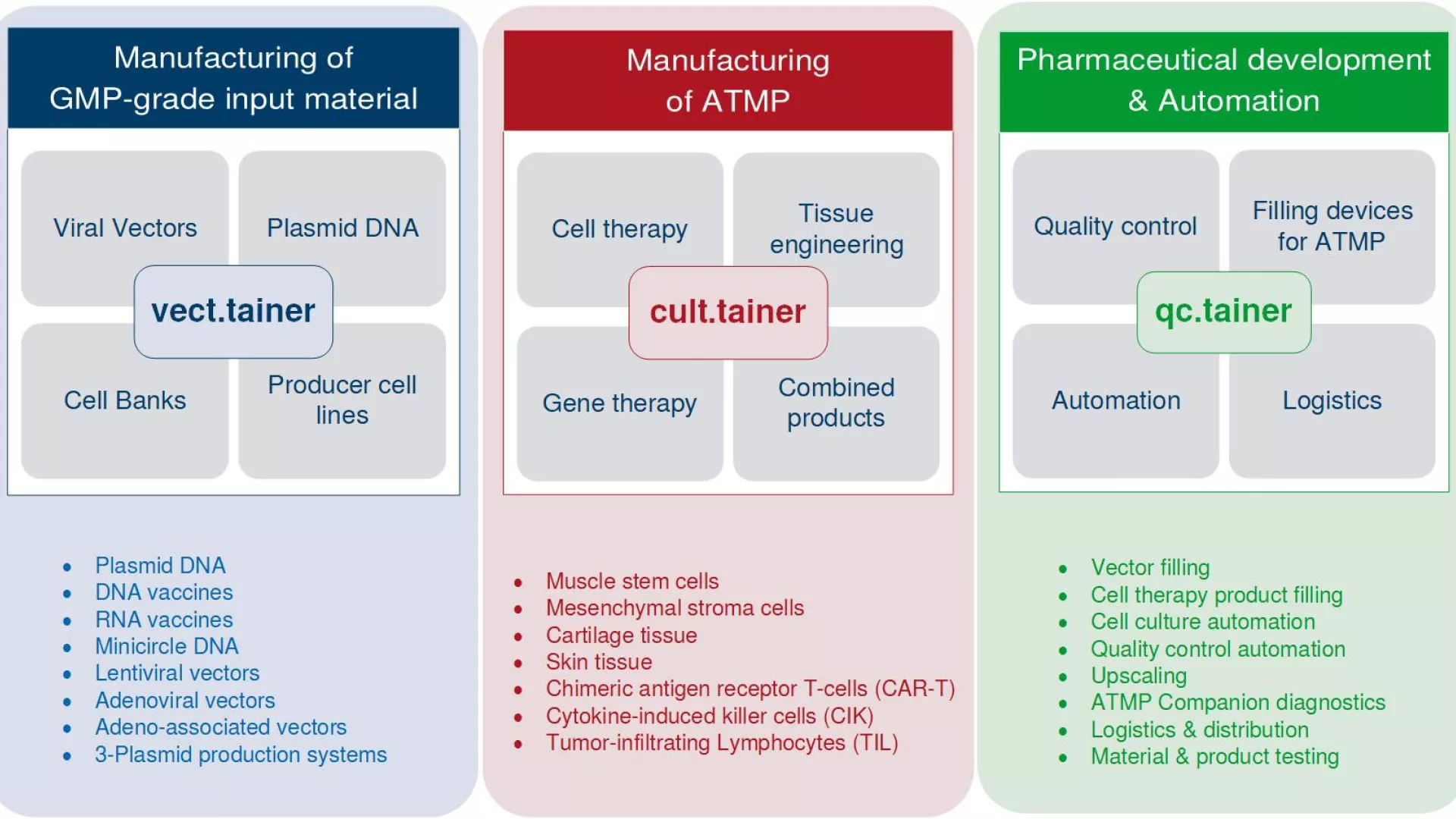
Valicare's cult.tainer concept primarily relates to the manufacture of ATMPs, while the overall valicare.tainer concept includes aspects such as the manufacture of starting materials, quality control, storage, automation, and filling. All modules can be combined to meet specific customer and process requirements.